Abstract
Multiple myeloma (MM) often occurs in middle-aged, elderly and obese patients with ectopic accumulation of fat cells in the bone marrow. Bone marrow adipocytes (BMAs) display unique immunomodulatory properties instead of simply providing energy substrates, which can cause distinct change of bone marrow microenvironment. Although BMA accounts for 70% of the total volume of bone marrow, the mechanism on how BMA affects tumor progression remains elusive. This study aims to explore the pathogenesis of BMA in promoting myeloma and new potential treatment strategies targeting bone marrow microenvironment.
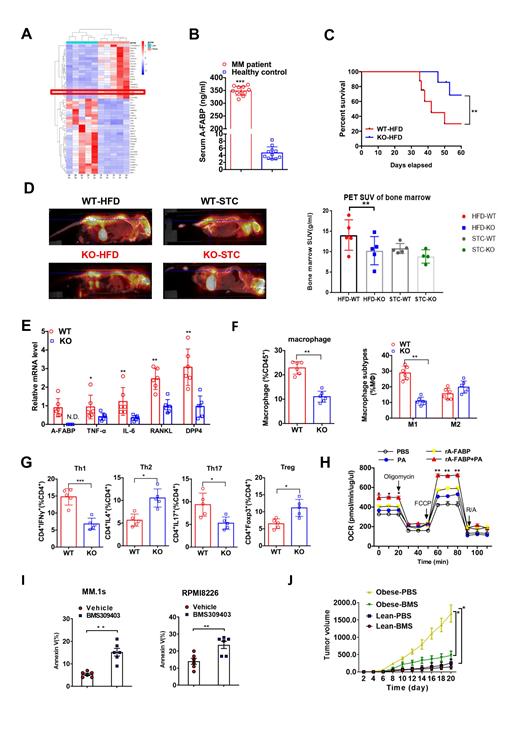
Newly diagnosed MM patients in our cancer center and their relative healthy controls are recruited. A significant increase of BMA quantity in multiple myeloma patients was observed. Moreover, analysis of transcriptome sequencing data of BMA derived from MM patients demonstrated a distinctive gene expression profiles (Fig A). It worth to note that, expression of fatty acid-binding protein 4 (FABP4, also known as A-FABP or aP2), a member of the FABP family abundantly expressed in adipocytes, functions as a lipid-binding chaperone that regulates trafficking and cellular signaling of fatty acids, and plays an important role in linking lipid metabolism with immunity and inflammation, was increased significantly in BMA of MM patients (Fig B). To further explore the role of FABP4 in pathogenesis in MM, FABP4 knockout (KO) mice and their wide-type (WT) littermates were adopted, and fed with standard chow (STC) or high-fat diet (HFD, 45 kcal % Fat, D12451). FABP4 deficiency significantly attenuated the tumor burden and MM-related osteolytic lesions in mice fed with HFD (Fig C-D). Moreover, levels of pro-inflammatory cytokines including TNFα, IL-6, RANKL and DPP4 were significantly reduced in FABP4 deficient adipocytes (Fig E). Flow cytometry analysis showed that the infiltration and pro-inflammatory polarization (M1/M2) of macrophages (MΦ) decreased significantly in FABP4 KO bone marrow (Fig F). In addition, FABP4 promoted the infiltration of Th1 and Th17 cells, while impaired the recruitment of Th2 and Treg cells (Fig G). Furthermore, administration of exogenous FABP4 recombinant protein significantly increased the fatty acid uptake and oxygen consumption of myeloma cells (Fig H). In contrast, pharmacological inhibition of FABP4 with BMS309403 alleviated the invasion and metastasis of MM in mice fed with HFD (Fig I-J).
In summary, BMA increased in MM patients, reshapes the metabolism and immunity in bone marrow microenvironment through regulating FABP4 functions. FABP4 enhanced the energy and lipid metabolism of myeloma cells, and manipulated the bone marrow microenvironment to pro-tumor environment, therefore promoted the proliferation and migration of myeloma cells. This study will not only clarify the critical role of BMA in MM pathogenesis, but also provide therapeutic potential of FABP4 selective inhibitor BMS309403 for multiple myeloma treatment, especially for obese MM patients.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal